
New Study Shows SEBiotic® Promotes Resolution of Constipation

New Study Shows SEBiotic® Promotes Resolution of Drug-Induced Constipation Associated with FGIDs
Forty percent of people around the world struggle with Functional Gastrointestinal Disorders (FGIDs).1 Constipation is a common, yet debilitating symptom associated with FGIDs, often exacerbated by prolonged use of certain medications.
A new, groundbreaking study reveals the therapeutic potential of the spore-forming probiotic SEBiotic® (Bacillus coagulans LBSC) in addressing drug-induced constipation associated with FGIDs.
Published in Global Advances in Integrative Medicine and Health, this clinical trial also underscores the efficacy and safety of SEBiotic®.
The Study
The double-blind, randomized, interventional, parallel-controlled clinical trial evaluated SEBiotic®’s impact on clinical symptoms, stool markers, safety and tolerability in patients suffering from drug-induced constipation linked to FGIDs.
The test group received SEBiotic® daily for 35 days, and the results were compared with those of the control group.
Key Findings
Results showed
-
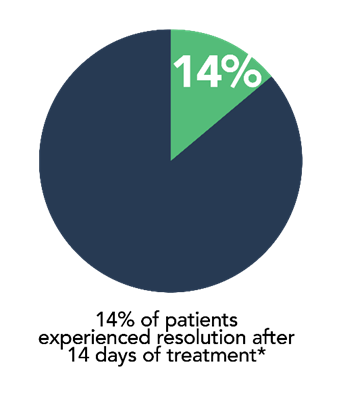
- 14% of patients experienced resolution of constipation after 14 days
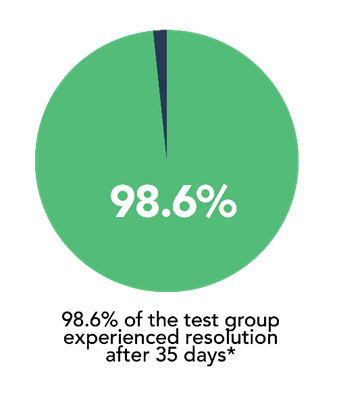
- 98.6% of patients experienced resolution of constipation after 35 days
- Improvement in stool consistency and expulsion, with a reduction in the occurrence of hard stools and defecation frequency
- SEBiotic® is safe for oral consumption, with no adverse events (AEs) or serious adverse events (SAEs) reported
Irritable Bowel Syndrome and Acute Diarrhea
The power of SEBiotic® goes far beyond addressing drug-induced constipation. Previous studies show its efficacy in promoting a reduction in symptoms of irritable bowel syndrome (IBS), acute diarrhea and abdominal discomfort, as well as its positive impact on gut microbiome modulation.
Regulatory Excellence
After an extensive scientific evidence evaluation, SEBiotic® received a GRAS (Generally Recognized as Safe) No Objections Letter from the FDA, ensuring its safety and efficacy.
Following a rigorous scientific review, Health Canada approved the following NPN (Natural Product Number) claims for SEBiotic®:
- Helps to relieve symptoms of irritable bowel syndrome (IBS), such as abdominal pain, bloating, cramping and nausea
- Helps support a healthy gut flora
- Source of probiotics
- Supports the maintenance of digestive health
Why SEBiotic® Stands Out
In addition to its regulatory excellence and 14 research studies, SEBiotic® is shelf and heat stable. It can survive processing and manufacturing and pass through the gastrointestinal tract in greater numbers to confer health benefits.
SEBiotic® is a resilient spore-forming probiotic that supports a balanced gut microbiome. Unlike conventional interventions that may have side effects or limited effectiveness, SEBiotic® offers clean-label and sustainable support for gastrointestinal issues.
Implications for FGID Management
The most recent study on SEBiotic® marks a significant milestone in addressing drug-induced constipation associated with FGIDs (functional gastrointestinal disorders). By supporting gut microbiota balance and a healthy gut, SEBiotic® offers an innovative approach to promoting relief and overall quality of life.
Takeaway
The new study results further confirm the power of SEBiotic® to support gut microbiota balance and gastrointestinal health. Its demonstrated ability to promote regular bowel movements, a resolution in constipation, bloating, diarrhea and abdominal discomfort—along with its resilience in processing and manufacturing and shelf stability—make it an ideal ingredient for supplements, functional foods and beverages.
-
Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J, Okeke E, Quigley EMM, Schmulson M, Whorwell P, Archampong T, Adibi P, Andresen V, Benninga MA, Bonaz B, Bor S, Fernandez LB, Choi SC, Corazziari ES, Francisconi C, Hani A, Lazebnik L, Lee YY, Mulak A, Rahman MM, Santos J, Setshedi M, Syam AF, Vanner S, Wong RK, Lopez-Colombo A, Costa V, Dickman R, Kanazawa M, Keshteli AH, Khatun R, Maleki I, Poitras P, Pratap N, Stefanyuk O, Thomson S, Zeevenhooven J, Palsson OS. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021 Jan;160(1):99-114.e3. doi: 10.1053/j.gastro.2020.04.014. Epub 2020 Apr 12. PMID: 32294476.




